
We place one electron in the orbital that is lowest in energy, the 1 s orbital. Unless there is a reason to show the empty higher energy orbitals, these are often omitted in an orbital diagram: Figure 2.1.1), and the electron configuration is written as 1 s 1 and read as “one-s-one.”Ī neutral helium atom, with an atomic number of 2 ( Z = 2), has two electrons. Some authors express the orbital diagram horizontally (removing the implicit energy axis and the colon symbol): Here is a schematic orbital diagram for a hydrogen atom in its ground state: A filled orbital is indicated by ↑↓, in which the electron spins are said to be paired. We use the orbital energy diagram of Figure 2.1.1, recognizing that each orbital can hold two electrons, one with spin up ↑, corresponding to m s = +½, which is arbitrarily written first, and one with spin down ↓, corresponding to m s = −½. First we determine the number of electrons in the atom then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle. We construct the periodic table by following the aufbau principle (from German, meaning “building up”). Thus, the columns of the periodic table represent the potential shared state of these elements' outer electron shells that is responsible for their similar chemical characteristics.\) When an atom gains an electron to become a negatively-charged ion this is indicated by a minus sign after the element symbol for example, \(F^-\). Group 17 elements, including fluorine and chlorine, have seven electrons in their outermost shells they tend to fill this shell by gaining an electron from other atoms, making them negatively-charged ions.
#Carbon electron configuration standard form plus
When an atom loses an electron to become a positively-charged ion, this is indicated by a plus sign after the element symbol for example, Na +. As a result of losing a negatively-charged electron, they become positively-charged ions. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron.
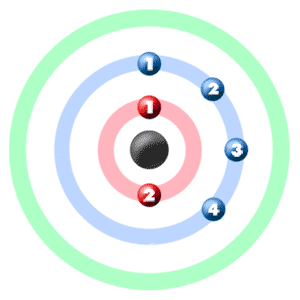
In comparison, the group 1 elements, including hydrogen (H), lithium (Li), and sodium (Na), all have one electron in their outermost shells. Their non-reactivity has resulted in their being named the inert gases (or noble gases). As shown in, the group 18 atoms helium (He), neon (Ne), and argon (Ar) all have filled outer electron shells, making it unnecessary for them to gain or lose electrons to attain stability they are highly stable as single atoms. The periodic table is arranged in columns and rows based on the number of electrons and where these electrons are located, providing a tool to understand how electrons are distributed in the outer shell of an atom.
#Carbon electron configuration standard form full
Elements in other groups have partially-filled valence shells and gain or lose electrons to achieve a stable electron configuration.Īn atom may gain or lose electrons to achieve a full valence shell, the most stable electron configuration.

A full valence shell is the most stable electron configuration. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. \):īohr diagrams indicate how many electrons fill each principal shell.


 0 kommentar(er)
0 kommentar(er)
